A computational study of ligand binding affinities in iron(iii) porphine and protoporphyrin IX complexes†
Abstract
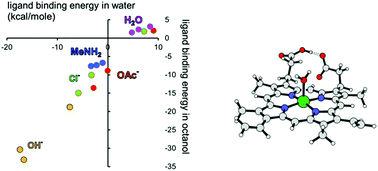
The search for novel anti-malarial drugs that can disrupt biomineralization of ferriprotoporphyrin IX to haemozoin requires an understanding of the fundamental chemistry of the porphyrin's iron(III) centre at the water–lipid interface. Towards this end, the binding affinities for a diverse set of 31 small ligands with iron(III) porphine have been calculated using density functional theory, in the gas phase and also with implicit solvent corrections for both water and n-octanol. In addition, the binding of hydroxide, chloride, acetate, methylamine and water to ferriprotoporphyrin IX has been studied, and very similar trends are observed for the smaller and larger models. Anionic ligands generally give stronger binding than neutral ones; the strongest binding is observed for RO− and OH− ligands, whilst acetate binds relatively weakly among the anions studied. Electron-rich nitrogen donors tend to bind more strongly than electron-deficient ones, and the weakest binding is found for neutral O and S donors such as oxazole and thiophene. In all cases, ligand binding is stronger in n-octanol than in water, and the differences in binding energies for the two solvents are greater for ionic ligands than for neutrals. Finally, dimerization of ferriprotoporphyrin IX by means of iron(III)–carboxylate bond formation has been modelled. The results are discussed in terms of haemozoin crystal growth and its disruption by known anti-malarial drugs.

 Please wait while we load your content...
Please wait while we load your content...