Self-assembly of a family of suprametallomacrocycles: revisiting an o-carborane bisterpyridyl building block†
Abstract
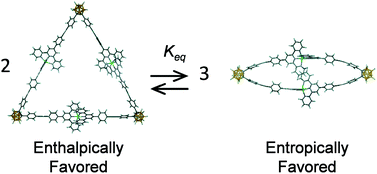
The self-assembly of the o-carborane-based, bisterpyridyl monomer, 1,2-bis[4′-(4-ethynylphenyl)-2,2′:6′,2′′-terpyridine]-o-carborane, utilizing either ZnII or FeII in a precise metal : ligand ratio (1 : 1), generated a family of metallomacrocycles that were studied via ESI-TWIM-MS, 1H NMR, and 2D NMR (COSY, NOESY). Under kinetic control, via formation of FeII complexes, the main cyclic product was triangular, as is typical of 60°-based bisligands. Under thermodynamic control using more labile transition metal complexes, e.g. ZnII, the ratio of cyclic species was found to be concentration and temperature dependent, and under an adequate entropic driving force, the cyclic dimer was formed. This system was probed via variable temperature NMR to reveal dynamic equilibrium between the entropically favored dimer and enthalpically favored trimer.

 Please wait while we load your content...
Please wait while we load your content...