Synthesis of di- and tetranuclear oxido-molybdenum(v) complexes containing p-toluenesulfonates as ligands: a joint spectroscopic, crystallographic and computational study†
Abstract
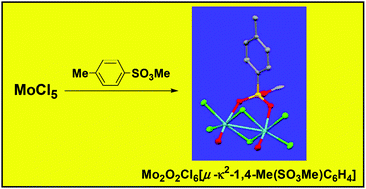
The 1 : 1 reaction of MoCl5 with 1,4-Me(SO3Me)C6H4 led to the isolation of crystalline Mo2O2Cl6[μ–κ2-1,4-Me(SO3Me)C6H4], 1, in low yields. Attempts to reproduce the synthesis of 1 from MoOCl3/1,4-Me(SO3Me)C6H4 resulted in the good-yield formation of [MoOCl2(μ-Cl)(κ1-1,4-Me(SO3Me)C6H4)]2, 2. The 1 : 1 reaction of MoCl5 with 1,4-Me(SO3H)C6H4·H2O selectively yielded Mo4O4Cl8[μ3-1,4-Me(SO3)C6H4]4, 3. Compounds 1 and 3 were characterized by X-ray diffractometry; moreover DFT calculations were carried out on 1–3 in order to shed light on their thermodynamic and structural features.

 Please wait while we load your content...
Please wait while we load your content...