Adsorption dominant catalytic activity of a carbon dots stabilized gold nanoparticles system†
Abstract
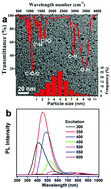
Carbon dots (CDs) with abundant functional groups (–OH, –COOH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) on their surface were specially designed to enhance the adsorption capacity and the catalytic activity of gold nanoparticles (AuNPs). The CDs stabilized gold nanoparticles (AuNPs-CDs) were greenly synthesized by a one-step reduction of HAuCl4 with CDs which were used as both the reductant and the stabilizer under visible light irradiation. The resulting AuNPs-CDs exhibit a high catalytic activity towards the reduction of 4-nitrophenol, with a good linear correlation of ln(Ci/C0) versus time and a kinetic rate constant about 0.68 min−1. Further detailed adsorption kinetics data indicated that the present adsorption system follows a predominantly second-order rate model, and the CDs capped on the surface of the AuNPs also enhanced the adsorption capacity and the catalytic activity of the AuNPs.
O) on their surface were specially designed to enhance the adsorption capacity and the catalytic activity of gold nanoparticles (AuNPs). The CDs stabilized gold nanoparticles (AuNPs-CDs) were greenly synthesized by a one-step reduction of HAuCl4 with CDs which were used as both the reductant and the stabilizer under visible light irradiation. The resulting AuNPs-CDs exhibit a high catalytic activity towards the reduction of 4-nitrophenol, with a good linear correlation of ln(Ci/C0) versus time and a kinetic rate constant about 0.68 min−1. Further detailed adsorption kinetics data indicated that the present adsorption system follows a predominantly second-order rate model, and the CDs capped on the surface of the AuNPs also enhanced the adsorption capacity and the catalytic activity of the AuNPs.

 Please wait while we load your content...
Please wait while we load your content...