Long range charge transfer in trimetallic mixed-valence iron complexes mediated by redox non-innocent cyanoacetylide ligands†
Abstract
The reaction of Fe(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)(dppe)Cp (1) with one-half equivalent of [trans-Fe(N
N)(dppe)Cp (1) with one-half equivalent of [trans-Fe(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe)2(dppx)2][BF4]2 (dppx = dppe ([2][BF4]2) or dppm ([3][BF4]2)) affords trimetallic [trans-Fe{N
CMe)2(dppx)2][BF4]2 (dppx = dppe ([2][BF4]2) or dppm ([3][BF4]2)) affords trimetallic [trans-Fe{N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CFe(dppe)Cp}2(dppx)2][BF4]2 (dppx = dppe [4][BF4]2; dppx = dppm [5][BF4]2). Both [4][BF4]2 and [5][BF4]2 undergo three, one-electron oxidation processes, arising from sequential oxidation of the two terminal Fe(C
CFe(dppe)Cp}2(dppx)2][BF4]2 (dppx = dppe [4][BF4]2; dppx = dppm [5][BF4]2). Both [4][BF4]2 and [5][BF4]2 undergo three, one-electron oxidation processes, arising from sequential oxidation of the two terminal Fe(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(dppe)Cp moieties and finally the central Fe(N
CR)(dppe)Cp moieties and finally the central Fe(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)2(dppx)2 fragment. The redox products [4]n+ and [5]n+ (n = 3, 4) have been characterised by UV-vis-NIR and IR spectroelectrochemistry. The shifts in the characteristic ν(C
CR)2(dppx)2 fragment. The redox products [4]n+ and [5]n+ (n = 3, 4) have been characterised by UV-vis-NIR and IR spectroelectrochemistry. The shifts in the characteristic ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
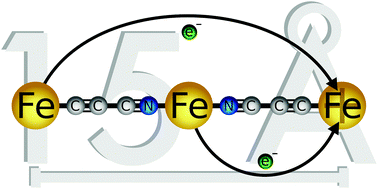
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) bands upon oxidation demonstrate not only the localised electronic structure of the trications, but also the redox non-innocence of the cyanoacetylide ligands. The trimetallic [formally Fe(II/II/III) mixed-valence] complexes [4]3+ and [5]3+ feature two distinct IVCT transitions, one associated with charge transfer from the central 18-electron {Fe(N
N) bands upon oxidation demonstrate not only the localised electronic structure of the trications, but also the redox non-innocence of the cyanoacetylide ligands. The trimetallic [formally Fe(II/II/III) mixed-valence] complexes [4]3+ and [5]3+ feature two distinct IVCT transitions, one associated with charge transfer from the central 18-electron {Fe(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)2(dppx)2}2+ to terminal {Fe(C
CR)2(dppx)2}2+ to terminal {Fe(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(dppe)Cp}+ moiety, and a lower energy transition involving charge transfer between the terminal Fe fragments which are separated by the redox active 9-atom, 10-bond –C
CR)(dppe)Cp}+ moiety, and a lower energy transition involving charge transfer between the terminal Fe fragments which are separated by the redox active 9-atom, 10-bond –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N{Fe(dppx)2}N
N{Fe(dppx)2}N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– bridge. The tetracationic complexes [4]4+ and [5]4+ generated by a further stepwise oxidation exhibit a single {Fe(N
C– bridge. The tetracationic complexes [4]4+ and [5]4+ generated by a further stepwise oxidation exhibit a single {Fe(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)2(dppx)2}2+→{Fe(C
CR)2(dppx)2}2+→{Fe(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(dppe)Cp}+ IVCT transition.
CR)(dppe)Cp}+ IVCT transition.

 Please wait while we load your content...
Please wait while we load your content...