A streamlined synthesis of extended thiophloroglucinol ligands and their trinuclear NiII3 complexes†
Abstract
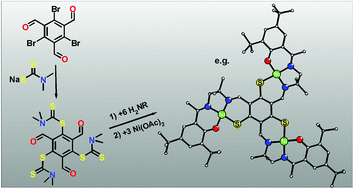
A protocol for the synthesis of trinucleating C3-symmetric ligands based on a central meta-phenylene bridging 1,3,5-trimercaptobenzene (thiophloroglucinol) backbone has been established. The key compound turned out to be the trialdehyde obtained from the triple nucleophilic attack of dimethyldithiocarbamate at 1,3,5-tribromo-2,4,6-triformylbenzene. Reacting this trialdehyde with six equivalents of a primary amine results in the simultaneous dithiocarbamate cleavage and Schiff-base formation providing the extended thiophloroglucinol ligands H3bertdien, H6bertMe, H6bertt-Bu2, and H6habbi. Reaction with NiII leads to the formation of the trinuclear NiII3 complexes [(bertdien)NiII3](X)3 (X = BPh4−, BF4−), [(bertMe)NiII3], [(bertt-Bu2)NiII3], and [(habbi)NiII3], which are characterized spectroscopically, electrochemically, and crystallographically.

 Please wait while we load your content...
Please wait while we load your content...