Facial triad modelling using ferrous pyridinyl prolinate complexes: synthesis and catalytic applications†
Abstract
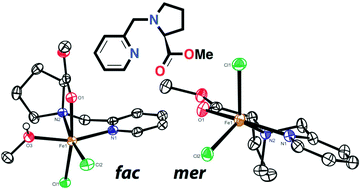
A series of new chiral pyridinyl prolinate (RPyProR) ligands and their corresponding Fe(II) triflate and chloride complexes are reported. The ligands possess an NN′O coordination motif, as found in the active site of non-heme iron enzymes with the so-called 2-His-1-carboxylate facial triad. The coordination behaviour of these ligands towards iron turned out to be dependent on the counter ion (chloride or triflate), the crystallization conditions (coordinating or non-coordinating solvents) and the presence of substituents on the ligand. In combination with Fe(II)(OTf)2, coordinatively saturated complexes of the type [Fe(L)2](OTf)2 are formed, in which the ligands adopt a meridional coordination mode. The use of FeCl2 in a non-coordinating solvent leads to 5-coordinated complexes [Fe(L)(Cl)2] with a meridional N,N′,O ligand. Crystallization of these complexes from a coordinating solvent leads to 6-coordinated [Fe(L)(solv)(Cl)2] complexes (solv = methanol or acetonitrile), in which the N,N′,O ligand is coordinated in a facial manner. For RPyProR ligands bearing a 6-Me substituent on the pyridine ring, solvent coordination and, accordingly, ligand rearrangement are prevented by steric constraints. The complexes were tested as oxidation catalysts in the epoxidation of alkene substrates in acetonitrile with hydrogen peroxide as the oxidant under oxidant limiting conditions. The complexes were shown to be especially active in the epoxidation of styrene type substrates (styrene and trans-beta-methylstyrene). In the best case, complex [Fe(6-Me-PyProNH2)Cl2] (15) allowed for 65% productive consumption of hydrogen peroxide toward epoxide and benzaldehyde products.

 Please wait while we load your content...
Please wait while we load your content...