The quenching of fluorescence as an indicator of donor-strength in meso arylethynyl BODIPYs†
Abstract
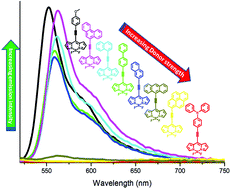
A series of meso arylethynyl BODIPYs (2a–2h) were designed and synthesized by the Pd-catalyzed Sonogashira cross-coupling reaction. The effects of the donor on the photophysical properties of the BODIPYs were explored. The DFT optimized structures and crystal structures show the planar orientation of the donor group with respect to the acceptor BODIPY, which favors a high degree of conjugation and induces strong donor–acceptor interactions. The quenching of fluorescence was correlated with the electron donating strength of the donor. The anthracene, pyrene and triphenylamine were found to have a stronger electron donating ability than the p-methoxyphenyl, phenanthrene, 1-naphthalene, biphenyl, and 2-naphthalene moieties. This was further supported by computational calculations and electrochemical analysis. The single crystal structures of BODIPYs 2d and 2e are reported, which show marvellous supramolecular structures.

 Please wait while we load your content...
Please wait while we load your content...