Non-symmetric diphosphines based on the imidazole scaffold: an unusual group interchange involving Pd–CH3 and (imidazole)P–Ph cleavage†
Abstract
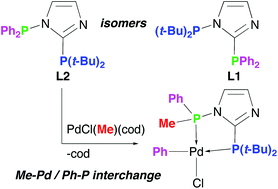
Two regioisomeric, non-symmetric PC2PN-imidazoles, t-Bu2PNCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNC(PPh2) (L1, PC2 = PPh2, PN = P(t-Bu)2) and Ph2PNCH
CHNC(PPh2) (L1, PC2 = PPh2, PN = P(t-Bu)2) and Ph2PNCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNC[P(t-Bu)2] (L2, PC2 = P(t-Bu)2, PN = PPh2), respectively, show dramatic differences in the reactivity of the N-bound phosphine group; the L2 isomer is extremely sensitive to P–N bond cleavage by nucleophiles, and when coordinated to the PdCl(Me) fragment it undergoes facile interchange of one PN phenyl with the methyl originating from Pd.
CHNC[P(t-Bu)2] (L2, PC2 = P(t-Bu)2, PN = PPh2), respectively, show dramatic differences in the reactivity of the N-bound phosphine group; the L2 isomer is extremely sensitive to P–N bond cleavage by nucleophiles, and when coordinated to the PdCl(Me) fragment it undergoes facile interchange of one PN phenyl with the methyl originating from Pd.

 Please wait while we load your content...
Please wait while we load your content...