Two new bismuth thiourea bromides: crystal structure, growth, and characterization†
Abstract
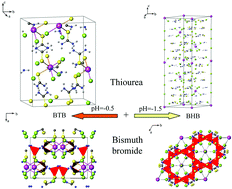
Crystals of two new bismuth thiourea bromides, bismuth trithiourea bromide (Bi[CS(NH2)2]3Br3, BTB) and bismuth protonated-hexathiourea bromide (Bi[CS(NH2)2H]6Br9, BHB), have been successfully grown from hydrobromic acid solution with different pH values by slow evaporation. Single crystal X-ray diffraction reveals that BTB is isostructural to its Cl-analog crystallizing in a monoclinic space group Cc with unit cell dimensions of a = 8.6238(7) Å, b = 12.2506(11) Å, c = 15.5040(13) Å, β = 90.7810(10)° and Z = 4. In contrast, BHB crystallizes in a trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c with unit cell dimensions of a = b = 12.748(17) Å, c = 40.45(11) Å, and Z = 6. The protonation of the thiourea in BHB is confirmed by the structure solution, IR and Raman spectroscopy. The UV diffuse reflection spectra clearly indicate that both of the two crystals have good optical transparency in the range below 2000 nm. Both compounds decompose above 190 °C, and BHB melts at around 140 °C while BTB possesses a phase transition at 145 °C as indicated by thermogravimetric (TG) and differential thermal analysis (DTA).
c with unit cell dimensions of a = b = 12.748(17) Å, c = 40.45(11) Å, and Z = 6. The protonation of the thiourea in BHB is confirmed by the structure solution, IR and Raman spectroscopy. The UV diffuse reflection spectra clearly indicate that both of the two crystals have good optical transparency in the range below 2000 nm. Both compounds decompose above 190 °C, and BHB melts at around 140 °C while BTB possesses a phase transition at 145 °C as indicated by thermogravimetric (TG) and differential thermal analysis (DTA).

 Please wait while we load your content...
Please wait while we load your content...