Intercalated supramolecular compounds of kaolinite with ethanolamine and ethylene glycol: structures and dielectric properties†
Abstract
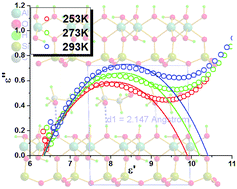
Kaolinite (K), a polar and layered aluminosilicate mineral, was used as the host; ethanolamine (EOA) and ethylene glycol (EG) were inserted into the kaolinite interlayer to give the intercalated supramolecular compounds kaolinite–ethanolamine (K-EOA) and kaolinite–ethylene glycol (K-EG), respectively. The intercalation of EOA and EG resulted in an increase in the d(001)-value by 3.4 and 3.68 Å, which corresponds to expansion of the interlayer space by 156.7 Å3 in K-EOA and 169.6 Å3 in K-EG, respectively. The characteristic infrared-active ν(O–H) modes ν1, ν2 and ν3 besides ν5, which were quite sensitive to the host–guest interaction, were not significantly affected by intercalation in K-EOA and K-EG, and two intercalated compounds showed lower deintercalation temperature (115 and 109 °C for K-EOA and K-EG, respectively). These are due to weakly intermolecular interactions between the intercalant molecules and the kaolinite framework, which is in agreement with the theoretical analysis of crystal structures of the intercalated compounds. K-EOA and K-EG showed novel dielectric relaxation behavior, which originates from the dynamic orientation motion of intercalant molecules.

 Please wait while we load your content...
Please wait while we load your content...