A calorimetric study of the hydrolysis and peroxide complex formation of the uranyl(vi) ion†
Abstract
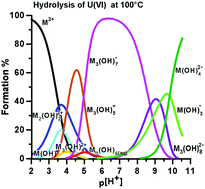
The enthalpies of reaction for the formation of uranyl(VI) hydroxide {[(UO2)2(OH)2]2+, [(UO2)3(OH)4]2+, [(UO2)3(OH)5]+, [(UO2)3(OH)6](aq), [(UO2)3(OH)7]−, [(UO2)3(OH)8]2−, [(UO2)(OH)3]−, [(UO2)(OH)4]2−} and peroxide complexes {[UO2(O2)(OH)]− and [(UO2)2(O2)2(OH)]−} have been determined from calorimetric titrations at 25 °C in a 0.100 M tetramethyl ammonium nitrate ionic medium. The hydroxide data have been used to test the consistency of the extensive thermodynamic database published by the Nuclear Energy Agency (I. Grenthe, J. Fuger, R. J. M. Konings, R. J. Lemire, A. B. Mueller, C. Nguyen-Trung and H. Wanner, Chemical Thermodynamics of Uranium, North-Holland, Amsterdam, 1992 and R. Guillaumont, T. Fanghänel, J. Fuger, I. Grenthe, V. Neck, D. J. Palmer and M. R. Rand, Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium, Elsevier, Amsterdam, 2003). A brief discussion is given about a possible structural relationship between the trinuclear complexes [(UO2)3(OH)n]6−n, n = 4–8.

 Please wait while we load your content...
Please wait while we load your content...