Surfactant behaviour of metallacarboranes. A study based on the electrolysis of water
Abstract
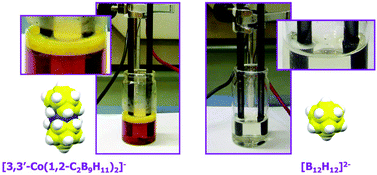
[3,3′-Co(1,2-C2B9H11)2]−, [1]−, and its chloroderivatives have been described as displaying surfactant/aggregation properties. We have studied their behaviour as electrolytes in the water electrolysis process. The electrolysis experiments support the surfactant behaviour of these compounds. These conclusions have been drawn on the grounds of the intensity/voltage (I/V) curves of water splitting into H2 and O2 of aqueous solutions in which the electrolytes have been tested at the same concentration. The I/V curves have permitted us to map and group the different electrolytes studied in this work. Three differentiated zones have been observed: one for true electrolytes, NO3− and ClO4−; a second one for intermediate electrolytes, BF4− and p-toluenesulfonate (PTS); and a third having the surfactant dodecylbenzenesulfonate (DBS), [1]− and its chloroderivatives. The incorporation into the study of the chloroderivatives of [1]− has allowed us to correlate molecular structure features with aqueous performance. The studied chloroderivatives perform better as electrolytes in accordance with the descending order of available B–H groups. This comes from the higher capacity to generate B–H⋯H–Cc dihydrogen bonds in the non- or less-halogenated molecules, considered one of the main reasons for the generation of the aggregates. In order to generate B–H⋯H–Cc dihydrogen bonds the H–Cc from the carborane cluster is needed. [B12H12]2− was chosen to prove the hypothesis as it has B–H units but lacks H–Cc units. Consequently, it should not produce self-assembling motifs, as is the case. [B12H12]2− has an aqueous behaviour similar to SO42−.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...