Triarylborane conjugated acacH ligands and their BF2 complexes: facile synthesis and intriguing optical properties†
Abstract
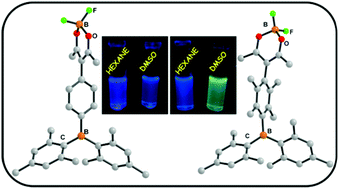
A facile synthetic route for a new class of organoborane compounds (Mes)2B-arene-acacH and (Mes)2B-arene-acacBF2 (Mes = mesityl and arene = C6H4 or C6Me4) is reported. The new dyads exhibit intriguing photophysical properties. A small structural change in spacer connecting the two chromophores leads to fine tuning of photophysical properties. The dyad containing 2,3,5,6-tetramethyl phenyl spacer acts as a selective “turn-on” chemodosimetric sensor for cyanide ion. Steric crowding around the boron centre significantly alters anion binding events. From NMR titration studies it is established that fluoride and cyanide follow different binding mechanisms which lead to intriguing optical properties in the reported probes.

 Please wait while we load your content...
Please wait while we load your content...