Methylene bridge regulated geometrical preferences of ligands in cobalt(iii) coordination chemistry and phenoxazinone synthase mimicking activity†
Abstract
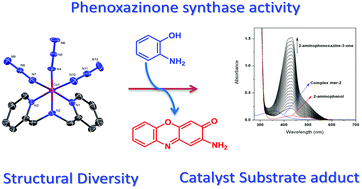
Two new azide bound cobalt(III) complexes, [Co(L1)(N3)3] (fac-1) and [Co(L2)(N3)3] (mer-2), where L1 is bis(2-pyridylmethyl)amine and L2 is (2-pyridylmethyl)(2-pyridylethyl)amine, derived from tridentate reduced Schiff-base ligands have been reported. Interestingly, a methylene bridge regulated preferential coordination mode of ligands is noticed in their crystal structures: it is found in a facial arrangement in fac-1 and has a meridional disposition in mer-2. Both complexes show phenoxazinone synthase-like activity and the role of the structural factor on the catalytic activity is also explored. Moreover, the easily reducible cobalt(III) center in mer-2 favors the oxidation of o-aminophenol. The ESI-MS positive spectra together with UV-vis spectroscopy clearly suggest the formation of a catalyst–substrate adduct by substitution of the coordinated azide ions in the catalytic cycle.

 Please wait while we load your content...
Please wait while we load your content...