Triarylborane–dipyrromethane conjugates bearing dual receptor sites: the synthesis and evaluation of the anion binding site preference†
Abstract
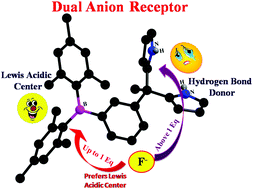
The synthesis and optical properties of four new triarylborane–dipyrromethane (TAB–DPM) conjugates (3a–d) containing dual binding sites (hydrogen bond donor and Lewis acid) have been reported. The new compounds exhibit a selective fluorogenic response towards the F− ion. The NMR titrations show that the anions bind to the TAB–DPM conjugates via the Lewis acidic triarylborane centre in preference to the hydrogen bond donor (dipyrromethane) units.

 Please wait while we load your content...
Please wait while we load your content...