Silver(i) supramolecular complexes generated from isophorone-based ligands: crystal structures and enhanced nonlinear optical properties through metal complexation†
Abstract
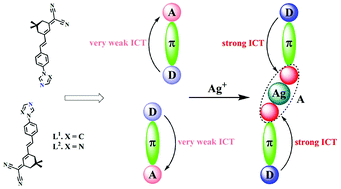
By self-assembly of (E)-2-(3-(4-(1H-imidazol-1-yl)styryl)-5,5-dimethylcyclohex-2-enylidene)malononitrile (L1) and (E)-2-(3-(4-(1H-1,2,4-triazol-1-yl)styryl)-5,5-dimethylcyclohex-2-enylidene)malononitrile (L2) with silver(I) salts, eight new complexes, namely AgL12ClO4 (1), AgL12NO3 (2), [AgL12NO3]·C6H6 (3), [AgL12OOCCF3]·C6H6 (4), [AgL12PF6]·C6H6 (5), AgL22NO3 (6), [AgL2OOCCF3]2 (7) and AgL22PF6 (8), are presented along with an analysis of their structural features. The structures are built up through the combination of coordination bonds, Ag⋯π, Ag⋯F (or O), hydrogen bonding, and π⋯π stacking interactions to generate new supramolecular architectures. We observed the formation of two-dimensional coordination polymers for complex 7. Solvent benzene molecules and anions are dispersed in the supramolecular structure and play a vital role in building the supramolecular structures of the complexes. The nonlinear optical (NLO) properties of the complexes were investigated using the Z-scan technique and complexes 1, 2, 3, 4 and 7 show obviously nonlinear absorption compared with ligands (L1 and L2).

 Please wait while we load your content...
Please wait while we load your content...