Compositional effects on the hydrogen storage properties of Mg(NH2)2–2LiH–xKH and the activity of KH during dehydrogenation reactions†
Abstract
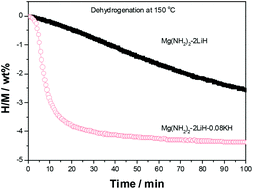
Potassium hydride (KH) was directly added to a Mg(NH2)2–2LiH system to improve the hydrogen storage properties; the corresponding mechanisms were elucidated. The Mg(NH2)2–2LiH–0.08KH composite displays optimized hydrogen-storage properties, reversibly storing approximately 5.2 wt% hydrogen through a two-stage reaction and a dehydrogenation onset at 70 °C. The 0.08KH-added sample fully dehydrogenated at 130 °C begins to absorb hydrogen at 50 °C, and takes up approximately 5.1 wt% of hydrogen at 140 °C. Adding KH significantly enhances the de-/hydrogenation kinetic properties; however, an overly rapid hydrogenation rate enlarges the particle size and raises the dehydrogenation temperature. A cycling evaluation reveals that the KH-added Mg(NH2)2–2LiH system possesses good reversible hydrogen storage abilities, although the operational temperatures for de-/hydrogenation increase during cycling. Detailed mechanistic investigations indicate that adding KH catalytically decreases the activation energy of the first dehydrogenation step and reduces the enthalpy of desorption during the second dehydrogenation step as a reactant, significantly improving the hydrogen storage properties of Mg(NH2)2–2LiH.

 Please wait while we load your content...
Please wait while we load your content...