Formation and reactivity of an (alkene)peroxoiridium(iii) intermediate supported by an amidinato ligand†
Abstract
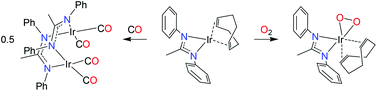
An IrI complex of an acetamidinato ligand was synthesized by reaction of N,N′-diphenylacetamidine, PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)NHPh, with either MeLi and [{Ir(cod)}2(μ-Cl)2] or [{Ir(cod)}2(μ-OMe)2] and was characterized by X-ray crystallography as a mononuclear complex, [Ir{PhNC(Me)NPh}(cod)] (1; where cod = 1,5-cyclooctadiene). Reaction of 1 with CO afforded a dinuclear carbonyl complex, [{Ir(CO)2}2{μ-PhNC(Me)NPh-κN:κN′}2] (2), as indicated by EI mass spectrometry and solution- and solid-state IR spectroscopy [νCO (n-pentane) = 2067, 2034 and 1992 cm−1]. Activation of O2 by 1 in solution at 20 °C was irreversible and produced an (alkene)peroxoiridium(III) intermediate, [Ir{PhNC(Me)NPh}(cod)(O2)] (3), which was characterized by one- and two-dimensional NMR techniques and IR spectroscopy (for 3, νOO = 860 cm−1; for 3–18O2, νOO = 807 cm−1). Complex 3 oxidized PPh3 to OPPh3, and its decay in the absence of added substrates followed by reaction with cod yielded 4-cycloocten-1-one and a minor amount of 1. In comparison with the results for the previously reported guanidinato complex [Ir{PhNC(NMe2)NPh}(cod)(O2)] (4), the formation of 3 and its reaction with PPh3 are significantly faster, indicating considerable ligand effects in these reactions.
C(Me)NHPh, with either MeLi and [{Ir(cod)}2(μ-Cl)2] or [{Ir(cod)}2(μ-OMe)2] and was characterized by X-ray crystallography as a mononuclear complex, [Ir{PhNC(Me)NPh}(cod)] (1; where cod = 1,5-cyclooctadiene). Reaction of 1 with CO afforded a dinuclear carbonyl complex, [{Ir(CO)2}2{μ-PhNC(Me)NPh-κN:κN′}2] (2), as indicated by EI mass spectrometry and solution- and solid-state IR spectroscopy [νCO (n-pentane) = 2067, 2034 and 1992 cm−1]. Activation of O2 by 1 in solution at 20 °C was irreversible and produced an (alkene)peroxoiridium(III) intermediate, [Ir{PhNC(Me)NPh}(cod)(O2)] (3), which was characterized by one- and two-dimensional NMR techniques and IR spectroscopy (for 3, νOO = 860 cm−1; for 3–18O2, νOO = 807 cm−1). Complex 3 oxidized PPh3 to OPPh3, and its decay in the absence of added substrates followed by reaction with cod yielded 4-cycloocten-1-one and a minor amount of 1. In comparison with the results for the previously reported guanidinato complex [Ir{PhNC(NMe2)NPh}(cod)(O2)] (4), the formation of 3 and its reaction with PPh3 are significantly faster, indicating considerable ligand effects in these reactions.

 Please wait while we load your content...
Please wait while we load your content...