Mass spectrometry and potentiometry studies of Pb(ii)–, Cd(ii)– and Zn(ii)–cystine complexes
Abstract
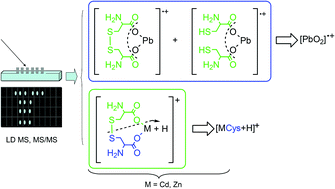
Cd(II)–, Pb(II)– and Zn(II)–cystine complexes were investigated by potentiometric and different mass spectrometric (MS) methodologies. Laser desorption mass spectrometry has provided both the composition and structure of metal–cystine complexes according to the speciation models proposed on the basis of the potentiometric data. Detection of neutral complexes was achieved by protonation or electrochemical reduction during mass spectrometric experiments. The redox activity of metal–cystine complexes was confirmed by laser desorption and charge transfer matrix assisted laser assisted MS experiments, which allowed us to observe the formation of complexes with a reduction of cystine. The stoichiometry of Cd(II)–, Pb(II)– and Zn(II)–cystine complexes was defined by observing the isotopic pattern of the investigated compound. The results suggest that interaction occurs through the carboxylate group of the ligand.

 Please wait while we load your content...
Please wait while we load your content...