Metal–organic frameworks built from achiral 3-(5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)benzoic acid: syntheses and structures of metal(ii) complexes†
Abstract
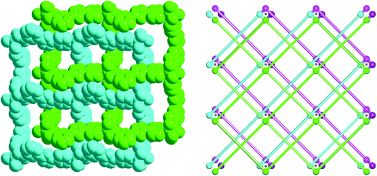
Using a novel flexible achiral ligand, 3-(5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)benzoic acid (HL), four metal(II)-complexes formulated as ZnL2·2H2O (1), CdL2(H2O)2·8H2O (2) and ML2(H2O)·H2O (M = Co 3 and Ni 4) have been hydrothermally synthesized and structurally characterized by single-crystal X-ray diffraction. Complexes 1, 3 and 4 all feature a uninodal 2D layer with a 44-sql topology, and two such (4,4) nets interpenetrate in a parallel manner. Complex 2 exhibits a similar 44-sql topology, but no interpenetration is observed in complex 2. Among the four complexes, only complex 1 is a homochiral network, which is evidenced by the CD spectrum. In complex 1, packing of the 2D layers generates a 41 screw axis along the c direction, and two-fold axes along the a and b directions, respectively. In complex 2, the Zn(II) center lies about an inversion center, giving rise to the centrosymmetric structure of complex 2. In complexes 3 and 4, packing of the 2D units generates a 21 screw axis along the c direction in the two complexes, and an inversion center is found between two neighboring 2-fold interpenetrated layers. The work indicates that the chirality of the space group for the homochiral complex 1 comes from the supramolecular packing of the 2D layers.

 Please wait while we load your content...
Please wait while we load your content...