Synthesis, structural characterization and photoluminescence property of three Zn2+/Mn2+-acylhydrazidate complexes and two acylhydrazide molecules†
Abstract
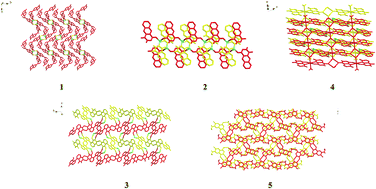
By employing the hydrothermal in situ acylation of N2H4 with aromatic polycarboxylic acids, three new acylhydrazidate-containing complexes [Zn(N2H4)(dphkh)]·H2O (dphkh = 4,4′-diphthalhydrazidatoketone hydrazone) 1, [Zn(npth)2] (npth = naphthalhydrazidate) 2 and [Mn(mpdh)2(H2O)2]·2H2O 4, and two new acylhydrazide molecules [bpth]·0.5H2O (bpth = 3,3′-biphthalhydrazide) 3 and [(chpth)2] (chpth = 4-chloro-5-hydrazinophthalhydrazide) 5 were obtained. It is noteworthy that (i) compound 1 is a layered Zn2+ coordination polymer with a mixed ligand of dphkh and N2H4. The nucleophilic addition of the keto spacer with N2H4 also occurred, forming the ketone hydrazone; (ii) compound 2 is a unique example of a npth-extended coordination polymer, exhibiting a double-chain structure; (iii) apart from the acylation of N2H4 with dcpha (dcpha = 4,5-dichlorophthalic acid), one Cl was substituted by N2H4, generating a new monoacylhydrazide molecule of compound 5. The solid-state photoluminescence analysis revealed that compounds 1 and 5 exhibit strong luminescence with the maximum at 490 nm for 1 and 535 nm for 5, whereas compounds 2 and 3 show weaker emissions with the peaks at 510 nm for 2 and 440 nm for 3.

 Please wait while we load your content...
Please wait while we load your content...