Peculiar properties of homoleptic Cu complexes with dipyrromethene derivatives†
Abstract
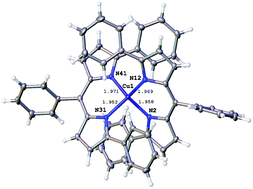
In view of preparing Cu polynuclear complexes with dipyrromethene ligands, the mononuclear complexes [Cu(II)(dipy)2] (dipyH = 5-phenyldipyrromethene) and [Cu(II)(dpdipy)2] (dpdipyH = 1,5,9-triphenyldipyrromethene) have been prepared and characterized by X-ray crystallography, mass spectrometry and EPR spectroscopy. Their peculiar redox and spectroscopic (absorption/emission) behaviours are discussed. In contrast to CuII complexes of 1,1′-bidypyrrin, the reduction electrolysis of [Cu(II)(dpdipy)2] leads to decomposition products on a time scale of a few hours. Moreover in relation to this observation, [Cu(I)(dpdipy)2]− could not be synthesized in spite of the CuI core protection by the phenyl substituents in ortho position of the nitrogen atoms. Theoretical calculations provide some explanations for this instability. Interestingly [Cu(II)(dipy)2] and [Cu(II)(dpdipy)2] display weak luminescence at room temperature, attributed to a ligand centered emission.

 Please wait while we load your content...
Please wait while we load your content...