Catalyst activity or stability: the dilemma in Pd-catalyzed polyketone synthesis†
Abstract
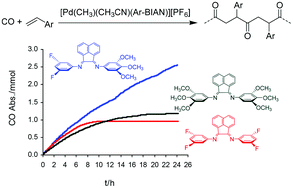
A series of Pd-complexes containing nonsymmetrical bis(aryl-imino)acenaphthene (Ar-BIAN)
* Corresponding authors
a
Dipartimento di Scienze Chimiche e Farmaceutiche, Università di Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy
E-mail:
milaniba@units.it
Fax: +39 040 5583903
Tel: +39 040 5583956
b
Dipartimento di Chimica, Fisica e Ambiente, Università di Udine, Via del Cotonificio 108, 33100 Udine, Italy
E-mail:
francesco.amoroso@uniud.it
Fax: +39 0432 558803
Tel: +39 0432 558755
c
Dipartimento di Scienze Biomolecolari, Università di Urbino, Piazza Rinascimento 6, 61029 Urbino, Italy
E-mail:
carla.carfagna@uniurb.it
Fax: +39 0722 303311
Tel: +39 0722 303312
d
Institut für Chemie und Biochemie-Anorganische Chemie, Freie Universität Berlin, 14195 Berlin, Germany
E-mail:
c.mueller@fu-berlin.de
Tel: +49 30 838 54004
e
School of Chemistry, University of Edinburgh, Edinburgh EH9 3JJ, Scotland, UK
E-mail:
d.vogt@ed.ac.uk
Tel: +44 (0)131 651 7767
f Chemistry Department, Alexandria University, Ibrahimia, Alexandria 21321, Egypt
g
Dipartimento di Chimica, Università di Milano, Via C. Golgi 19, 20133 Milano, Italy
E-mail:
fabio.ragaini@unimi.it
Fax: +39 02 50314405
Tel: +39 02 50314373
A series of Pd-complexes containing nonsymmetrical bis(aryl-imino)acenaphthene (Ar-BIAN)

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
F. Amoroso, E. Zangrando, C. Carfagna, C. Müller, D. Vogt, M. Hagar, F. Ragaini and B. Milani, Dalton Trans., 2013, 42, 14583 DOI: 10.1039/C3DT51425K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
