Oxidation reactions of stable chalcogenamides with iodine are intriguing due to their broad application in various organic syntheses. In the present study we report on the utilization of N-heterocyclic carbene and cyclic-alkyl-amino carbenes L1–3: (L1: = :C[N(2,6-iPr2-C6H3)CH]2, L2: = :C(CH2)(CMe2)(C6H10)N-2,6-iPr2-C6H3, L3: = :C(CH2)(CMe2)2N-2,6-iPr2-C6H3) for the syntheses of chalcogenamides L1–3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E (E = S, Se, Te) 1–4 and zwitterionic adducts L1–3
E (E = S, Se, Te) 1–4 and zwitterionic adducts L1–3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E–I–I 5–8. The synthesis of compounds 1–4 involved the addition reaction of ligand L1–3: and elemental chalcogen. Treatment of 1–4 with iodine resulted in the formation of adducts 5–8. Compounds 5–8 are well characterized with various spectroscopic methods and single-crystal X-ray structural analysis.
E–I–I 5–8. The synthesis of compounds 1–4 involved the addition reaction of ligand L1–3: and elemental chalcogen. Treatment of 1–4 with iodine resulted in the formation of adducts 5–8. Compounds 5–8 are well characterized with various spectroscopic methods and single-crystal X-ray structural analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E (E = S, Se, Te) 1–4 and zwitterionic adducts L1–3
E (E = S, Se, Te) 1–4 and zwitterionic adducts L1–3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E–I–I 5–8. The synthesis of compounds 1–4 involved the addition reaction of
E–I–I 5–8. The synthesis of compounds 1–4 involved the addition reaction of 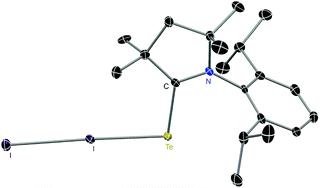

 Please wait while we load your content...
Please wait while we load your content...