Reactivity differences between 2,4- and 2,5-disubstituted zirconacyclopentadienes: a highly selective and general approach to 2,4-disubstituted phospholes†
Abstract
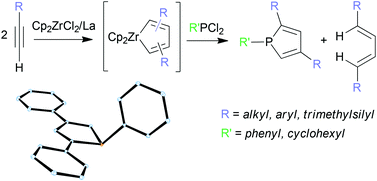
Mixtures of 2,4- and 2,5-disubstituted zirconacyclopentadienes were obtained by the reductive dimerisation of terminal

 Please wait while we load your content...
Please wait while we load your content...