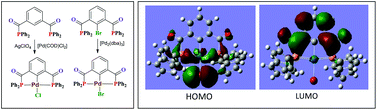
The bisphosphomide, 1,3-{Ph2PC(O)}2C6H4 (1), was prepared by the reaction of isophthaloyl chloride with diphenylphosphine in the presence of triethylamine. The corresponding bromo-derivative, 2-Br-1,3-{Ph2PC(O)}2C6H3 (2), was obtained by the reaction of 2-bromoisophthaloyl chloride with diphenylphosphine. The reaction of 1 with elemental sulfur or selenium yielded the bis(chalcogenides), 1,3-{Ph2P(S)C(O)}2C6H4 (3) and {1,3-Ph2P(Se)C(O)}2C6H4 (4). The reaction between 1 and [Ru(η6-p-cymene)Cl2]2 and [Pd(η3-C3H5)Cl]2 in 1 : 1 stoichiometry yielded the corresponding binuclear complexes, [Ru2(η6-p-cymene)2Cl4{1,3-{Ph2PC(O)}2(C6H4)}] (5) and [Pd2(η3-C3H5)2Cl2{1,3-{Ph2PC(O)}2(C6H4)}] (6). The reaction of 1 with AgClO4 followed by the addition of [Pd(COD)Cl2] at room temperature resulted in the formation of a pincer complex [PdCl{2,6-{Ph2PC(O)}2(C6H3)}] (9), via transmetallation. Pincer complex formation through C–H activation requires drastic conditions and yields are generally moderate. The oxidative addition reaction between 2 and [Ni(COD)2] gave a pincer complex [NiBr{2,6-{Ph2PC(O)}2(C6H3)}] (8), whereas the 2 : 1 reaction of 2 with [Pd2(dba)3] yielded the palladium analogue [PdBr{2,6-{Ph2PC(O)}2(C6H3)}] (9) in quantitative yield. The reaction between 1 and CuX in a 1 : 1 molar ratio produced binuclear complexes, [Cu2(μ-X)2{1,3-{Ph2PC(O)}2(C6H4)}2] (10, X = Cl; 11, X = Br; 12, X = I), whereas the reaction between 1 and [Cu(NCCH3)4]BF4 led to the isolation of a spirocyclic complex, [Cu(CH3CN)2{1,3-{Ph2PC(O)}2(C6H4)}]BF4 (13). The silver complexes [Ag2(μ-ClO4)2{1,3-{Ph2PC(O)}2(C6H4)}2] (14), [Ag2(μ-OTf)2{1,3-{Ph2PC(O)}2(C6H4)}2] (15) and [Ag2X2{1,3-{Ph2PC(O)}2(C6H4)}] (16, X = ClO4; 17, X = OTf) were obtained by treating 1 with AgClO4 or AgOTf in 1 : 1 or 1 : 2 molar ratios. The reactions of 1 with [AuCl(SMe2)] in 1 : 1 and 1 : 2 molar ratios afforded mono- and binuclear complexes, [AuCl{1,3-{Ph2PC(O)}2(C6H4)}2] (18) and [Au2Cl2{1,3-{Ph2PC(O)}2(C6H4)}AuCl] (19), in good yield. The structures of complexes 5, 7–10, 12 and 14a were confirmed by single-crystal X-ray diffraction studies. DFT calculations were performed in order to gain additional insights into the structure and bonding of the pincer complexes. An additional analysis of the orbital interactions in the case of palladium complex 9 is also included. The in situ generated rhodium complex of bisphosphomide 1 showed moderate to good selectivity in the hydroformylation of hex-1-ene and styrene derivatives.

 Please wait while we load your content...
Please wait while we load your content...