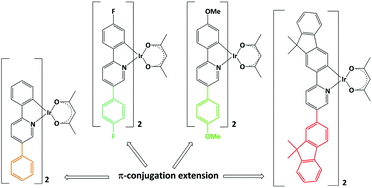
This manuscript reports on the synthesis, the photophysical study and the electroluminescent properties of a series of heteroleptic cyclometalated iridium(III) complexes based on 2,5-diaryl-pyridines as C^N cyclometalating ligands and acetylacetonate as ancillary ligand. The complexes were characterised by elemental analysis, ESI-MS, multinuclear NMR, TGA and electrochemistry. Their optical properties were investigated by UV-Vis and photoluminescence. DFT and TD-DFT calculations provided further insights into the effects of the 5-aryl substitution on the electronic and photophysical properties of the new complexes. The presence of suitable π-extended ligands exerts a beneficial effect on the performances of the corresponding solution-processed light-emitting diodes, leading to a maximum brightness of 10 620 cd m−2 at a current efficiency of 10.0 cd A−1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...