A series of novel binuclear phenoxyimino organoaluminum complexes of the type [(RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H3OAlMe2]2 [R = C6H5 (2a), 2,6-iPr2C6H3 (2b), 2,6-Ph2C6H3 (2c), adamantyl (2d), tBu (2e)] have been prepared in high yields, and these complexes were identified by 1H, 13C NMR and elemental analysis. Structural analysis for 2a–e revealed that these complexes have a distorted tetrahedral geometry around Al and both the Al–O and the Al–N bond distances were considerably influenced by substituents in the imino groups. The complexes were tested as catalyst precursors for ring-opening polymerisation (ROP) of ε-caprolactone (CL) in the presence of BnOH, and their catalytic activities were strongly affected by the catalyst structures and polymerisation conditions. An efficient living ROP has been achieved using the 2b/BnOH system.
CH)C6H3OAlMe2]2 [R = C6H5 (2a), 2,6-iPr2C6H3 (2b), 2,6-Ph2C6H3 (2c), adamantyl (2d), tBu (2e)] have been prepared in high yields, and these complexes were identified by 1H, 13C NMR and elemental analysis. Structural analysis for 2a–e revealed that these complexes have a distorted tetrahedral geometry around Al and both the Al–O and the Al–N bond distances were considerably influenced by substituents in the imino groups. The complexes were tested as catalyst precursors for ring-opening polymerisation (ROP) of ε-caprolactone (CL) in the presence of BnOH, and their catalytic activities were strongly affected by the catalyst structures and polymerisation conditions. An efficient living ROP has been achieved using the 2b/BnOH system.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H3OAlMe2]2 [R = C6H5 (2a), 2,6-iPr2C6H3 (2b), 2,6-Ph2C6H3 (2c), adamantyl (2d), tBu (2e)] have been prepared in high yields, and these complexes were identified by 1H,
CH)C6H3OAlMe2]2 [R = C6H5 (2a), 2,6-iPr2C6H3 (2b), 2,6-Ph2C6H3 (2c), adamantyl (2d), tBu (2e)] have been prepared in high yields, and these complexes were identified by 1H, 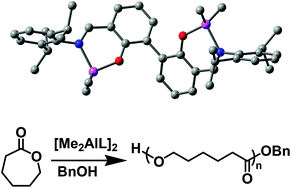

 Please wait while we load your content...
Please wait while we load your content...