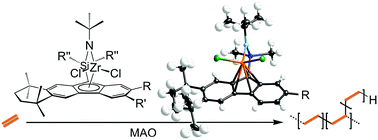
Several analogues of the sterically expanded constrained geometry catalyst Me2Si(η1-C29H36)(η1-N-tBu)ZrCl2·OEt2 (2) were synthesized to assess the effect on branching and molecular weight for ethylene homopolymerization. Catalysts based on tetramethyltetrahydrobenzofluorene (TetH), ethylTetH, t-butylTetH, and octamethyloctahydrodibenzofluorenyl (OctH) bearing a diphenylsilyl bridge were prepared and characterized: Me2Si(η5-C21H22)(η1-N-tBu)ZrCl2 (3); Me2Si(η5-C23H26)(η1-N-tBu)ZrCl2 (4); and Me2Si(η5-C25H30)(η1-N-tBu)ZrCl2 (5); Me2Si(η5-C21H22)(η1-N-tBu)ZrMe2 (6); and Ph2Si(η5-C29H36)(η1-N-tBu)ZrCl2 (7). Complexes 4, 5, 6, and 7 were characterized by X-ray crystallography and displayed η5 hapticity to the carbon ring in each case, in contrast to 2. In comparison to 2, complexes 3, 4, 5, and 7 (in combination with methylaluminoxane = MAO) showed diminished branching, higher molecular weight, and higher polydispersity indices for obtained ethylene homopolymers. While 4/MAO produced the greatest molecular weight polymers, no branching was observed. Reactivity ratios were determined for the copolymerization of ethylene and 1-decene with 2/MAO. A value of rethylene = 14.9 and an exceedingly high value of r1-decene = 0.49 were found—in line with previous reports of this catalyst's unusual affinity for α-olefins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...