Neutral tricoordinated beryllium(0) compounds – isostructural to BH3 but isoelectronic to NH3†
Abstract
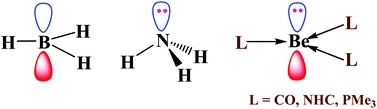
The electronic structure and reactivity of neutral tricoordinated Be(0) compounds BeL3, L = CO (1), NHC (2) and PMe3 (3) are explored by quantum mechanical calculations. These BeL3 complexes are found to be planar or nearly planar like electron deficient BH3 but isoelectronic with

 Please wait while we load your content...
Please wait while we load your content...