Effects of Al-based additives on the hydrogen storage performance of the Mg(NH2)2–2LiH system†
Abstract
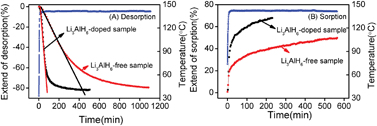
The Mg(NH2)2–2LiH composite is a promising hydrogen storage material due to its relatively high reversible hydrogen capacity (∼5.6 wt%) and suitable thermodynamic properties that allow hydrogen

 Please wait while we load your content...
Please wait while we load your content...