A dinuclear manganese(III) complex (1) of an N-(carboxymethyl)-N-[3,5-bis(α,α-dimethylbenzyl-2-hydroxybenzyl)]glycine (HDA) ligand (L) binds a manganese(II) species through displacement of its solvating ligands by appropriately dispositioned carbonyl groups of a dinuclear complex {[Mn2(L)2(OH)(OCH3)][Mn(H2O)3(CH3OH)3], 2, triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.172(3) Å, b = 15.897(3) Å, c = 19.059(4) Å, V = 3461.9(13) Å3} leading to a trinuclear complex {3, monoclinic P21/n, a = 11.7606(8) Å, b = 21.3505(8) Å, c = 26.7827(17) Å, V = 6722.7(7) Å3} with cyclization of two of the carboxy groups through the doubly-carboxy group coordinated Mn2+ ion. The reaction is discussed in terms of its significance as an illustration of how Mn2+ ions might be sequestered in biological systems. A similar solvato-ligand displacement reaction was used to synthesise coordination polymers of an HDA iron(III) complex involving polymerization through a bridging carboxylato group. Several isostructural polymers (5–7; for 5: orthorhombic Pbca, a = 9.411(5) Å, b = 16.390(8) Å, c = 37.968(19) Å, V = 5856(5) Å3) with different coordinated alcohols could be prepared indicating the potential synthetic uses of this method.
, a = 13.172(3) Å, b = 15.897(3) Å, c = 19.059(4) Å, V = 3461.9(13) Å3} leading to a trinuclear complex {3, monoclinic P21/n, a = 11.7606(8) Å, b = 21.3505(8) Å, c = 26.7827(17) Å, V = 6722.7(7) Å3} with cyclization of two of the carboxy groups through the doubly-carboxy group coordinated Mn2+ ion. The reaction is discussed in terms of its significance as an illustration of how Mn2+ ions might be sequestered in biological systems. A similar solvato-ligand displacement reaction was used to synthesise coordination polymers of an HDA iron(III) complex involving polymerization through a bridging carboxylato group. Several isostructural polymers (5–7; for 5: orthorhombic Pbca, a = 9.411(5) Å, b = 16.390(8) Å, c = 37.968(19) Å, V = 5856(5) Å3) with different coordinated alcohols could be prepared indicating the potential synthetic uses of this method.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.172(3) Å, b = 15.897(3) Å, c = 19.059(4) Å, V = 3461.9(13) Å3} leading to a trinuclear complex {3, monoclinic P21/n, a = 11.7606(8) Å, b = 21.3505(8) Å, c = 26.7827(17) Å, V = 6722.7(7) Å3} with
, a = 13.172(3) Å, b = 15.897(3) Å, c = 19.059(4) Å, V = 3461.9(13) Å3} leading to a trinuclear complex {3, monoclinic P21/n, a = 11.7606(8) Å, b = 21.3505(8) Å, c = 26.7827(17) Å, V = 6722.7(7) Å3} with 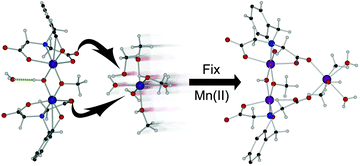

 Please wait while we load your content...
Please wait while we load your content...