BODIPY-modified Ru(ii) arene complex—a new ligand dissociation mechanism and a novel strategy to red shift the photoactivation wavelength of anticancer metallodrugs†
Abstract
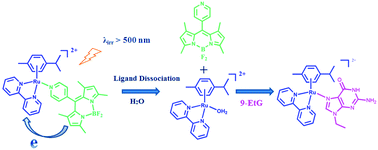
A Ru(II) arene complex [(η6-p-cymene)Ru(bpy)(py-BODIPY)](PF6)2, where bpy is 2,2′-bipyridine and py-BODIPY is a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene dye containing a pyridine group at the 8-position, was designed and synthesized. BODIPY modification renders the monodentate pyridine ligand with long wavelength absorbing capability, and an absorption maximum at 504 nm. Upon selective irradiation of the absorption band of the py-BODIPY ligand, the dissociation of the monodentate ligand occurs efficiently, followed by substitution by 9-ethylguanine if it is present in the solution. The photoinduced ligand dissociation quantum yield was measured to be 4.1% at 480 nm. The photoinduced electron transfer from the BODIPY chromophore to the Ru(II) arene moiety plays an important role in the ligand dissociation. Such a photosensitization strategy can be utilized to develop novel anticancer metallodrugs that may respond to light in the phototherapeutic window (650–900 nm).

 Please wait while we load your content...
Please wait while we load your content...