Glycosylated copper(ii) ionophores as prodrugs for β-glucosidase activation in targeted cancer therapy†
Abstract
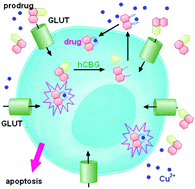
8-Hydroxyquinoline derivatives are metal-binding compounds that have recently attracted interest as therapeutic agents for cancer therapy. In this scenario, we designed and synthesized three new glucoconjugates, 5,7-dichloro-8-quinolinyl-β-D-glucopyranoside, 5-chloro-8-quinolinyl-β-D-glucopyranoside and 2-methyl-8-quinolinyl-β-D-glucopyranoside and investigated their biological properties in comparison to the parent 8-hydroxyquinoline derivatives in the presence of Cu2+. In vitro data show that 2 out of 3 glycosylated compounds possess a pharmacologically-relevant antiproliferative activity against tumor cells, similar to that of their parent compounds; this activity is associated with a relevant triggering of apoptosis. The pharmacological profile of the glucoconjugates depends on the cellular enzymatic β-glucosidase activity, as demonstrated by the inhibition of antiproliferative activity in the presence of the 2,5-dideoxy-2,5-imino-D-mannitol.

 Please wait while we load your content...
Please wait while we load your content...