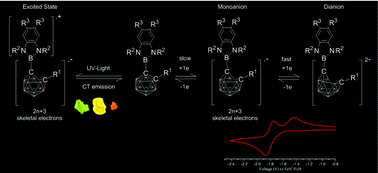
Fifteen C-diazaborolyl-ortho-carboranes, 1-R′-2-R′′-1,2-C2B10H10, where R′ represents the groups 2-(1,3-Et2-1,3,2-N2BC6H4)-, 2-(1,3-Ph2-1,3,2-N2BC6H4)-, 2-(1,3-Ph2-5,6-Me2-1,3,2-N2BC6H2)-, 2-(1,3-iPr2-1,3,2-N2BC6H4)-, and 2-(1,3,2-N2BC6H6)- and where R′′ is H, Me, Ph, tBu or SiMe3, were synthesized. Cyclic voltammetry studies of the compounds showed irreversible oxidation waves which are caused by the oxidation of the heterocycle. Those C-diazaborolyl-ortho-carboranes with Ph, tBu and SiMe3 substituents at the adjacent C-atom of the cage displayed two one-electron reduction waves reflecting the formation of stable radical monoanions with unusual (2n + 3) skeletal electron counts. The geometries of these anions were determined by combinations of infrared, UV-visible spectroelectrochemical and computational studies. Additionally the structures of seven new C-diazaborolyl-ortho-carboranes and one new 2-bromo-1,3,2-benzodiazaborole were determined by X-ray crystallography and compared with previously obtained structures.

 Please wait while we load your content...
Please wait while we load your content...