PVP stabilized Pt nano particles catalyzed de-oxygenation of phenoxazine group by hydrazine in physiological buffer media: surfactant competes with reactants for the same surface sites†
Abstract
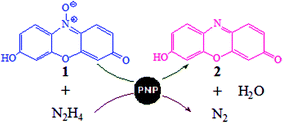
PVP capped platinum nano particles (PNP) of 5 nm diameter were prepared and characterized as homogeneous and of spherical nature. At physiological pH range (6.0–8.0), these PNP catalyze the deoxygenation of phenoxazine group containing resazurin (1) by hydrazine. The observed rate constants (ko), increase linearly with [PNP] at constant [1] and [Hydrazine]; but first increase and then after reaching a maximum it decrease with increase in [1] as well as in [Hydrazine]. The ko values increase linearly with 1/[H+] indicating N2H4 as the reducing species that generates from the PNP assisted deprotonation of N2H5+. The kinetic observations suggest Langmuir–Hinshelwood type surface reaction mechanism where both 1 and hydrazine are adsorbed on nano particles surface and compete for the same sites. Interestingly, the surfactant molecules, polyvinylpyrrolidone (PVP), though do not take part into reduction reaction but having same type of functional groups as reactants, competes with them for the same surface sites. Adsorption on PNP with same type of functional group is further supported by the FTIR spectra of Pt-PVP and Pt-1. Thus on increasing [PVP], ko decreases linearly and only when [PVP] is held constant, the plot of kovs. [PNP] passes through the origin indicating the insignificance of uncatalyzed reaction. The plot of ln kovs. [1] or [Hydrazine] shows two different linear zones with different exponent values with respect to [1] and [Hydrazine]. This indicates that along with the complex heterogeneous surface adsorption processes, the mutual interactions between the reactants are also changing with the relative concentrations of reactants or, in general, with the molar ratio ([Hydrazine]/[1]).

 Please wait while we load your content...
Please wait while we load your content...