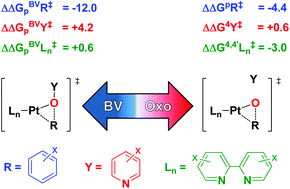
A computational Hammett analysis of oxy-insertion into platinum–aryl bonds is performed. Modeled transformations involve the two-step conversion of [(Xbpy)Pt(R)(OY)]+ (R = p- or m-X-C6H4; Y = 4- or 3-X-pyridine; Xbpy = 4,4′- or 5,5′-X-bpy; X = NO2, H, OMe, NMe2) proceeding through a Pt–oxo intermediate to form aryloxide [(Xbpy)Pt(OR)(Y)]+, which contrasts a one-step non-redox (Baeyer–Villiger) oxy-insertion. A structural connection is proposed between redox and non-redox transition states, linked to, among other parameters, oxidant identity. The electronic impact of the catalytic components is compared to previous Hammett studies on OMBV transformations. The Hammett sensitivity for aryl migration is diminished for the migrating group (R) and leaving group (Y), components as compared to OMBV transitions, while the bipyridine supporting ligand (Ln) has an increased impact. The Hammett impact of R, Y and Ln upon the aryl migration transition state is small in a global sense, ca. 5 kcal mol−1; therefore, we conclude that the metal and oxidant are the most important factors in controlling oxy-insertion kinetics for these late metal systems. These results also point to a possible mechanistic advantage for redox over non-redox functionalization of hydrocarbons to alcohols.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...