Effects of mutating aromatic surface residues of the heme domain of human sulfite oxidase on its heme midpoint potential, intramolecular electron transfer, and steady-state kinetics†
Abstract
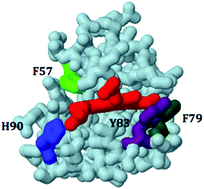
Human sulfite oxidase (hSO), an essential molybdoheme enzyme, catalyzes the oxidation of toxic sulfite to sulfate. The proposed catalytic cycle includes two, one-electron intramolecular electron transfers (IET) between the molybdenum (Mo) and the heme domains. Rapid IET rates are ascribed to conformational changes that bring the two domains into close proximity to one another. Previous studies of hSO have focused on the roles of conserved residues near the Mo active site and on the tether that links the two domains. Here four aromatic surface residues on the heme domain (phenylalanine 57 (F57), phenylalanine 79 (F79), tyrosine 83 (Y83), and histidine 90 (H90)) have been mutated, and their involvement in IET rates, the heme midpoint potential, and the catalytic activity of hSO have been investigated using laser flash photolysis, spectroelectrochemistry, and steady-state kinetics, respectively. The results indicate that the size and hydrophobicity of F57 play an important role in modulating the heme potential and that F57 also affects the IET rates. The data also suggest that important interactions of H90 with a heme propionate group destabilize the Fe(III) state of the heme. The positive charge on H90 at pH ≤ 7.0 may decrease the electrostatic interaction between the Mo and heme domains, thereby decreasing the IET rates of wt hSO at low pH. Lastly, mutations of F79 and Y83, which are located on the surface of the heme domain, but not in direct contact with the heme or the propionate groups, have little effect on either IET or the heme potential.
- This article is part of the themed collection: Bioinorganic chemistry

 Please wait while we load your content...
Please wait while we load your content...