Lewis acidic behavior of B(C6Cl5)3†
Abstract
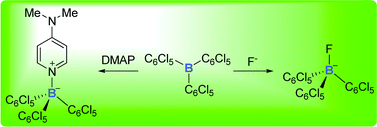
We report that B(C6Cl5)3 is a potent Lewis acid which, despite the bulk of the pentachlorophenyl group, forms complexes with small anions, including fluoride, or organic Lewis bases, such as
- This article is part of the themed collection: Boranes and borohydrides

 Please wait while we load your content...
Please wait while we load your content...