Single-molecule magnets based on rare earth complexes with chelating benzimidazole-substituted nitronyl nitroxide radicals†
Abstract
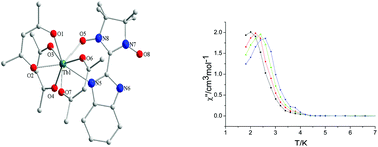
Three rare earth–nitronyl nitroxide radical complexes [Ln(tfa)3(NIT-BzImH)] (Ln(III) = Gd 1, Tb 2, Dy 3; tfa = trifluoroacetylacetonate; NIT-BzImH = 2-(2′-benzimidazolyl)-4,4,5,5-tetramethylimidazolyl-1-oxyl-3-oxide) have been successfully prepared and structurally characterized.

 Please wait while we load your content...
Please wait while we load your content...