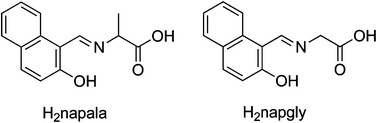
Two novel bpy-bridged CoII Schiff base complexes have been synthesized by the hydro(solvo)thermal reactions of corresponding amino-acid-based Schiff bases, bpy and Co(NO3)2·6H2O. The following formulae identify the two complexes: {[Co(napala)(bpy)0.5]·H2O}n (1) and [Co(napgly)(bpy)0.5]n (2) [H2napala = N-(2-hydroxy-1-naphthylmethylidene)-D/L-alanine, H2napgly = N-(2-hydroxy-1-naphthylmethylidene)-glycine and bpy = 4,4′-bipyridine]. These two compounds have been characterized using single-crystal X-ray diffraction, infrared, powder X-ray diffraction, thermogravimetric analysis, optical spectra analysis, and magnetic measurement. Complex 1 features an unprecedented threefold interpenetrated diamond network based on the fan-shaped CoII4(μ2-napala)4 molecular square node and bpy linker, which represents the first example of 3D framework among the amino-acid-based Schiff base complexes with salicylaldehyde or its derivatives. In 2, adjacent CoII ions are bridged by μ2-napgly2− to form left- and right-handed [CoII(μ2-napgly)]n helical chains. These two types of helical chains are sustained alternately by a symmetrical bpy co-ligand into a 2D grid-based layer. The solid-state fluorescence of complexes 1 and 2 are quenched almost completely compared with free mixed-ligands at room temperature. Moreover, magnetic studies show the dominant antiferromagnetic coupling between the CoII centers mediated by the syn–anti-COO−-bridges in both complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...