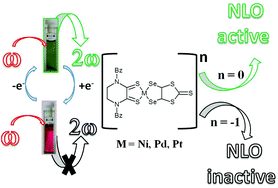
The donor–acceptor type mixed-ligand complexes [M(Bz2pipdt)(dsit)]; dsit = 2-thioxo-1,3-dithiole-4,5-diselenolato (donor); Bz2pipdt = 1,4-dibenzyl-piperazine-2,3-dithione (acceptor); M(II) = Ni (1), Pd (2), and Pt (3) were prepared and characterized to investigate the variation of the properties by substituting selenium for sulfur in the donor ligand dmit = 2-thioxo-1,3-dithiole-4,5-dithiolato of the corresponding known complexes. Both these classes of complexes exhibit large negative second-order polarizabilities, amongst the highest values determined so far for metal-complexes, and are potential candidates for redox switchability of the molecular first hyperpolarizability due to the bleaching/restoring of the solvatochromic peak for mono-reduction/oxidation. DFT and TD-DFT calculations on 1–3 allow one to correlate geometries and electronic structures and are in agreement with the observed minor changes following the substitution of selenium for sulfur atoms in the dichalcogenolato ligand. The observed differences can be ascribed to the increased size of the selenium atom leading to increased M–X distances and dipolar moments of the ground state, which are highest for the Pd-derivative in the triad.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...