The reaction of the 16-electron half-sandwich complex MeCpCo(S2C2B10H10) (1b; MeCp = methylcyclopentadienyl) and methyl propiolate (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me) at ambient temperature leads to MeCpCo(S2C2B10H9)(CH
CCO2Me) at ambient temperature leads to MeCpCo(S2C2B10H9)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (2), MeCpCo(S2C2B10H8)(CH
CHCO2Me) (2), MeCpCo(S2C2B10H8)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)2 (3), MeCpCo(S2C2B10H9)[MeO2CC
CHCO2Me)2 (3), MeCpCo(S2C2B10H9)[MeO2CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(MeO2C)C
CH(MeO2C)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)](CH
CH)](CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (4) and MeCpCo(S2C2B10H9)(CH2CCO2Me) (5). The reaction of Me4CpCo(S2C2B10H10) (1c; Me4Cp = tetramethylcyclopentadienyl) and the alkyne gives rise to Me4CpCo(S2C2B10H10)[MeO2CC
CHCO2Me) (4) and MeCpCo(S2C2B10H9)(CH2CCO2Me) (5). The reaction of Me4CpCo(S2C2B10H10) (1c; Me4Cp = tetramethylcyclopentadienyl) and the alkyne gives rise to Me4CpCo(S2C2B10H10)[MeO2CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(MeO2C)C
CH(MeO2C)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH] (6) and Me4CpCo (S2C2B10H9)(CH2CCO2Me) (7). Both 2 and 3 are 16-electron complexes but containing a B(3)-substituted o-carborane-1,2-dithiolate ligand in 2 and a B(3,6)-disubstituted o-carborane-1,2-dithiolate ligand in 3, respectively. In 4 and 6, two alkynes are inserted into one Co–S bond to meet an 18 electron configuration at metal, however, 4 has one B-substitution at carborane. Both 5 and 7 have the same structural type bearing a B–CH2 unit. The reactions of Cp#Co(E2C2B10H10) [Cp# = Cp (1a), MeCp (1b), Me4Cp (1c) and Me5Cp (1d); E = S, Se] with 2-methylpropanedithioic acid (L1) or pyrrolidine-1-carbodithioic acid (L2) lead to Co[S2CCH(CH3)2]3 (8) or Co[S2CN(CH2)4]3 (9), respectively, in an octahedral geometry. The three-component reactions of 1a–1d, methyl propiolate and L1 or L2 afford seven new compounds Cp#Co[S2C2B10H10(CH
CH] (6) and Me4CpCo (S2C2B10H9)(CH2CCO2Me) (7). Both 2 and 3 are 16-electron complexes but containing a B(3)-substituted o-carborane-1,2-dithiolate ligand in 2 and a B(3,6)-disubstituted o-carborane-1,2-dithiolate ligand in 3, respectively. In 4 and 6, two alkynes are inserted into one Co–S bond to meet an 18 electron configuration at metal, however, 4 has one B-substitution at carborane. Both 5 and 7 have the same structural type bearing a B–CH2 unit. The reactions of Cp#Co(E2C2B10H10) [Cp# = Cp (1a), MeCp (1b), Me4Cp (1c) and Me5Cp (1d); E = S, Se] with 2-methylpropanedithioic acid (L1) or pyrrolidine-1-carbodithioic acid (L2) lead to Co[S2CCH(CH3)2]3 (8) or Co[S2CN(CH2)4]3 (9), respectively, in an octahedral geometry. The three-component reactions of 1a–1d, methyl propiolate and L1 or L2 afford seven new compounds Cp#Co[S2C2B10H10(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)][S2CCH(CH3)2] [Cp# = Cp (10a), MeCp (10b) and Me4Cp (10c)], [S2CCH(CH3)2]2Co(S2C2B10H10)(CH
CHCO2Me)][S2CCH(CH3)2] [Cp# = Cp (10a), MeCp (10b) and Me4Cp (10c)], [S2CCH(CH3)2]2Co(S2C2B10H10)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCO2Me){CpCo[S2CCH(CH3)2]} (11a), Cp#Co[S2C2B10H10(CH
CCO2Me){CpCo[S2CCH(CH3)2]} (11a), Cp#Co[S2C2B10H10(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)][S2CN(CH2)4] [Cp# = Cp (12a), MeCp (12b) and Me4Cp (12c)]. All 10a–10c and 12a–12c contain one deprotonated L1 or L2 ligand and one reduced alkyne. 11a has two 18-electron Co centers linked by one reduced alkyne. One metal is coordinated by an o-carborane-1,2-dithiolate and two L1 ligands, and the other is coordinated by one L1 ligand and one η5-Cp unit. In both two- and three-component reactions the reactivity of the 16-electron half-sandwich complexes Cp#Co(S2C2B10H10) is dependent on the size of the Cp# unit. All compounds were fully characterized by spectroscopic techniques and elemental analysis. The solid-state structures of 4, 5, 7, 10a and 11a were further determined by X-ray crystallographic analysis.
CHCO2Me)][S2CN(CH2)4] [Cp# = Cp (12a), MeCp (12b) and Me4Cp (12c)]. All 10a–10c and 12a–12c contain one deprotonated L1 or L2 ligand and one reduced alkyne. 11a has two 18-electron Co centers linked by one reduced alkyne. One metal is coordinated by an o-carborane-1,2-dithiolate and two L1 ligands, and the other is coordinated by one L1 ligand and one η5-Cp unit. In both two- and three-component reactions the reactivity of the 16-electron half-sandwich complexes Cp#Co(S2C2B10H10) is dependent on the size of the Cp# unit. All compounds were fully characterized by spectroscopic techniques and elemental analysis. The solid-state structures of 4, 5, 7, 10a and 11a were further determined by X-ray crystallographic analysis.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me) at ambient temperature leads to MeCpCo(S2C2B10H9)(CH
CCO2Me) at ambient temperature leads to MeCpCo(S2C2B10H9)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (2), MeCpCo(S2C2B10H8)(CH
CHCO2Me) (2), MeCpCo(S2C2B10H8)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)2 (3), MeCpCo(S2C2B10H9)[MeO2CC
CHCO2Me)2 (3), MeCpCo(S2C2B10H9)[MeO2CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(MeO2C)C
CH(MeO2C)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)](CH
CH)](CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me) (4) and MeCpCo(S2C2B10H9)(CH2CCO2Me) (5). The reaction of Me4CpCo(S2C2B10H10) (1c; Me4Cp = tetramethylcyclopentadienyl) and the
CHCO2Me) (4) and MeCpCo(S2C2B10H9)(CH2CCO2Me) (5). The reaction of Me4CpCo(S2C2B10H10) (1c; Me4Cp = tetramethylcyclopentadienyl) and the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(MeO2C)C
CH(MeO2C)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH] (6) and Me4CpCo (S2C2B10H9)(CH2CCO2Me) (7). Both 2 and 3 are 16-electron complexes but containing a B(3)-substituted o-carborane-1,2-dithiolate
CH] (6) and Me4CpCo (S2C2B10H9)(CH2CCO2Me) (7). Both 2 and 3 are 16-electron complexes but containing a B(3)-substituted o-carborane-1,2-dithiolate ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)][S2CCH(CH3)2] [Cp# = Cp (10a), MeCp (10b) and Me4Cp (10c)], [S2CCH(CH3)2]2Co(S2C2B10H10)(CH
CHCO2Me)][S2CCH(CH3)2] [Cp# = Cp (10a), MeCp (10b) and Me4Cp (10c)], [S2CCH(CH3)2]2Co(S2C2B10H10)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCO2Me){CpCo[S2CCH(CH3)2]} (11a), Cp#Co[S2C2B10H10(CH
CCO2Me){CpCo[S2CCH(CH3)2]} (11a), Cp#Co[S2C2B10H10(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Me)][S2CN(CH2)4] [Cp# = Cp (12a), MeCp (12b) and Me4Cp (12c)]. All 10a–10c and 12a–12c contain one deprotonated L1 or L2
CHCO2Me)][S2CN(CH2)4] [Cp# = Cp (12a), MeCp (12b) and Me4Cp (12c)]. All 10a–10c and 12a–12c contain one deprotonated L1 or L2 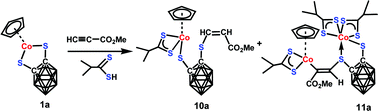

 Please wait while we load your content...
Please wait while we load your content...