Synthesis, structure and luminescence properties of Cu(ii), Zn(ii) and Cd(ii) complexes with 4′-terphenylterpyridine†
Abstract
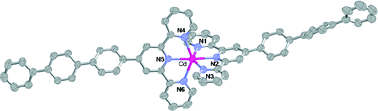
The complexes [M(tptpy)2](ClO4)2 (M = Zn(II) (1), Cd(II) (2), and Cu(II) (3)); tptpy = 4′-[1,1′:4′,1′′]terphenyl-4′′-yl-[2,2′:6′,2′′]terpyridine = 4′-terphenylterpyridine) have been synthesized, structurally characterized by X-ray crystallography and subjected to preliminary luminescence studies. In the crystalline state, all the metal ions have an N6 coordination sphere of distorted octahedral geometry and the structures of the Zn(II) and Cd(II) complexes are isomorphous but differ from that of the Cu(II) complex, which also differs from the other two in that it is non-emissive. The structure determinations show that aromatic–aromatic interactions involving both the terpyridine heads and the terphenyl tails are important factors influencing the crystalline array. The emission spectra of the Zn(II) and Cd(II) complexes are very similar and show a considerable red-shift of the emission maximum compared to that of the free ligand.

 Please wait while we load your content...
Please wait while we load your content...