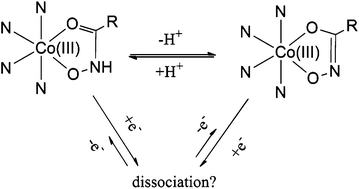
Extensive research is currently being conducted into metal complexes that can selectively deliver cytotoxins to hypoxic regions in tumours. The development of pharmacologically suitable agents requires an understanding of appropriate ligand–metal systems for chaperoning cytotoxins. In this study, cobalt complexes with tripodal tren (tris-(2-aminoethyl)amine) and tpa (tris-(2-pyridylmethyl)amine) ligands were prepared with ancillary hydroxamic acid, β-diketone and catechol ligands and several parameters, including: pKa, reduction potential and cytotoxicity were investigated. Fluorescence studies demonstrated that only tpa complexes with β-diketones showed any reduction by ascorbate in situ and similarly, cellular cytotoxicity results demonstrated that ligation to cobalt masked the cytotoxicity of the ancillary groups in all complexes except the tpa diketone derivative [Co(naac)tpa](ClO4)2 (naac = 1-methyl-3-(2-naphthyl)propane-1,3-dione). Additionally, it was shown that the hydroxamic acid complexes could be isolated in both the hydroxamate and hydroximate form and the pKa values (5.3–8.5) reveal that the reversible protonation/deprotonation of the complexes occurs at physiologically relevant pHs. These results have clear implications for the future design of prodrugs using cobalt moieties as chaperones, providing a basis for the design of cobalt complexes that are both more readily reduced and more readily taken up by cells in hypoxic and acidic environments.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...