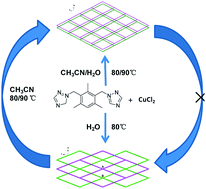
A nonporous neutral framework [CuCl2(m-bttmb)2]n (1) was changed into a porous ionic {[Cu(m-bttmb)2(H2O)Cl]Cl(CH3CN)0.5(H2O)2.75}n (2) by simply increasing the amount of CH3CN in the mixed solvent (CH3CN and H2O) or temperature in the reactions of CuCl2·2H2O with 1,3-bis(triazol-1-ylmethyl)-2,4,6-trimethylbenzene (m-bttmb). 1 undergoes transformation into 2 when treated with CH3CN. Both 1 and 2 have 2D 4-connected (4,4) network architectures but in different packing arrangements. These compounds have been characterized by single-crystal X-ray diffraction analysis, elemental analysis, IR spectra and thermogravimetric analysis. This work may provide a way to control the formation of neutral or ionic frameworks, as well as porosities by adjusting the polarity and components of the solvents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...