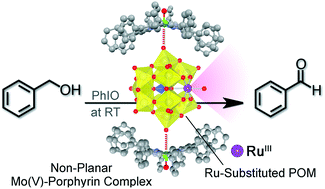
A 2 : 1 complex composed between a non-planar Mo(V)–porphyrin complex ([Mo(DPP)(O)]+, DPP2− = dodecaphenylporphyrin) and a ruthenium-substituted Keggin-type heteropolyoxometalate (Ru-POM), [SiW11O39RuIII(DMSO)]5−, acts as an efficient catalyst for oxidation of benzyl alcohols with iodosobenzene as an oxidant in CDCl3 at room temperature. The catalytic oxidation afforded the corresponding benzaldehydes, whereas neither the ammonium salt of Ru-POM nor [Mo(DPP)(O)]+ alone exhibited catalytic reactivity under the same experimental conditions. This enhancement can be attributed to a large anodic shift of the redox potential of the ruthenium centre due to the complexation of the Ru-POM with two cationic {Mo(DPP)(O)}+ units. The kinetic analysis demonstrated that the catalytic oxidation proceeded via formation of a catalyst-substrate complex, and electron-withdrawing substituents at the para position of benzyl alcohol accelerated the reaction. The rate constants of the oxidation reactions correlate to the bond dissociation energies of the C–H bonds of the substrate. A linear correlation was observed for logarithm of the rate constants of oxidation reactions of benzyl alcohols with that of hydrogen abstraction by cumyl peroxyl radical, indicating the reaction proceeds via hydrogen abstraction. The observed kinetic isotope effect (KIE) indicates that the hydrogen abstraction occurs from the benzyl group rather than the hydroxy group.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...