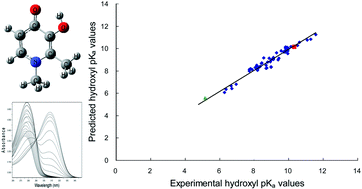
As an aid in optimising the design of 3-hydroxypyridin-4-ones (HPOs) intended for use as therapeutic Fe3+ chelating agents, various quantum mechanical (QM) and semi-empirical (QSAR) methods have been explored for predicting the pKa values of the hydroxyl groups in these compounds. Using a training set of 15 HPOs with known hydroxyl pKa values, reliable predictions are shown to be obtained with QM calculations using the B3LYP/6-31+G(d)/CPCM model chemistry (with Pauling radii, and water as solvent). With this methodology, the observed hydroxyl pKa values for the training set compound are closely matched by the predicted pKa values, with the correlation between the observed and predicted values giving r2 = 0.98. Predictions subsequently made by this method for a test set of 48 HPOs of known hydroxyl pKa values (11 of which were determined experimentally in this study), gave predicted pKa values accurate to within ±0.2 log units. In order to further investigate the predictive power of the method, two novel HPOs were synthesised and their hydroxyl pKa values were determined experimentally. Comparison of these predicted pKa values against the measured values gave absolute deviations of 0.13 (10.18 vs. 10.31) and 0.43 (5.58 vs. 5.15).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...