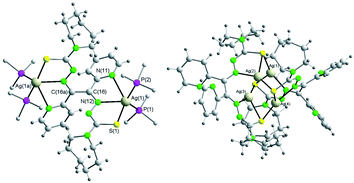
The reaction of pyridylbis(3-hexamethyleneiminyl thiosemicarbazone) (H2Plhexim) with various silver(I) salts and metal–ligand ratios led to the isolation of different complexes of the formulae [Ag(NO3)(H2Plhexim)]·H2O (1), [Ag2(NO3)(H2Plhexim)(CH3OH)](NO3) (2), [Ag2(ClO4)2(H2Plhexim)] (3), [Ag(HPlhexim)]·xH2O (4), [Ag(HPlhexim)] (4a), [Ag2(Plhexim)(PPh3)4]·2MeOH (5) and [Ag4(Plhexim)2]·DMF (6). The complexes were fully characterized by elemental analysis, ESI mass spectrometry, IR and NMR (1H, 31P) spectroscopy. The structures of 4a, 5 and 6 were also identified by single crystal X-ray structure determination. The concentration dependence on the absorption spectra of the methanolic solutions indicates polymerization equilibria in the ground state in both the ligand and the complexes. While H2Plhexim is essentially non-fluorescent, complexes 1–5 fluoresce more strongly by comparison. This fluorescent behavior is consistent with the monomeric or dimeric nature of the complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...